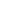F
Click on the icon info to get details.
Species data
| Common Formula | F |
| Stoichiometric Formula | F |
| Name | Fluorine atom |
| Mass | 18.99840 a.m.u |
| Charge | 0 |
| CAS | 14762-94-8 |
| Inchi | InChI=1S/F |
| InchiKey | YCKRFDGAMUMZLT-UHFFFAOYSA-N |
| State | Ground State
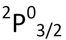
|

ISM Abundance
| log10 Abundance | Reference | Source Name | Source Type | Link |
|---|
Polarizability
| Evaluation | Definition | Value (Å3) | Method | Origin | Reference |
|---|---|---|---|---|---|
| total | 0.554 ±0.011 | Reviews and Evaluations | Bibliography | Schwerdtfeger, Peter et al. ;2019;Molecular Physics;117, 1200-1225 | |
| total | 0.557 ±0.01114 | Calculations | Bibliography | Lide, D. R.. ;2000; |
|
Definition: total Value (Å3): 0.554 ±0.011 Method: Reviews and Evaluations Origin: Bibliography Reference: Schwerdtfeger, Peter et al. ;2019;Molecular Physics;117, 1200-1225 |
|
Definition: total Value (Å3): 0.557 ±0.01114 Method: Calculations Origin: Bibliography Reference: Lide, D. R.. ;2000; |
Dipole moment
| Evaluation | Value (D) | Method | Origin | Reference |
|---|---|---|---|---|
| 0 | N/A | Database : NIST COMPUTATIONAL CHEMISTRY COMPARISON AND BENCHMARK DATABASE |
|
Value (D):
0 Method: N/A Origin: Database : NIST COMPUTATIONAL CHEMISTRY COMPARISON AND BENCHMARK DATABASE Reference: |
Enthalpy of formation
| Evaluation | T (K) | Value (kJ.mol-1) | Method | Origin | Reference |
|---|---|---|---|---|---|
| 0 | 77.274 ±0.058 | Reviews and Evaluations | Database : Burcat | ||
| 298 | 79.39 ±0.058 | Reviews and Evaluations | Database : Burcat | ||
| 298 | 79.38 ±0.3 | Measurements | Database : CCCBDB (http://cccbdb.nist.gov/) | ||
| 0 | 77.27 ±0.3 | Measurements | Database : CCCBDB (http://cccbdb.nist.gov/) |
|
T (K): 0
Value (kJ.mol-1) : 77.274 ±0.058 Method: Reviews and Evaluations Origin: Other database Reference: |
|
T (K): 298
Value (kJ.mol-1) : 79.39 ±0.058 Method: Reviews and Evaluations Origin: Other database Reference: |
|
T (K): 298
Value (kJ.mol-1) : 79.38 ±0.3 Method: Measurements Origin: Other database Reference: |
|
T (K): 0
Value (kJ.mol-1) : 77.27 ±0.3 Method: Measurements Origin: Other database Reference: |
Desorption energy
| Evaluation | Emean (K) | Emin (K) | Emax (K) | Pre-exponential factor (s-1) | Order factor | Method | Origin | Reference | Type of surface | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| 800 | 0 | 0 | 0.00E+0 | 1 | Estimation | Database : OSU | H2O | This binding energy was listed in the original OSU gas-grain code from Eric Herbst group in 2006. The energy is the same as O The pre-exponential factor is not given. It can be computed using the formula given in Hasegawa et al. (1992). |
|
Emean (K): 800
E min (K): 0 E max (K): 0 Pre-exponential factor (s-1): 0.00E+0 Method: Estimation Origin: Other database Reference: Type of surface: H2O Description: This binding energy was listed in the original OSU gas-grain code from Eric Herbst group in 2006. The energy is the same as O The pre-exponential factor is not given. It can be computed using the formula given in Hasegawa et al. (1992). Evaluation: |
Diffusion energy
No data