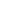N + NH
Gaz phase reaction (type: Bimolecular reactions)
Datasheet T(K) = 10-500
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NH
→
H + N2
|
10-500 | Modified Arrhenius equation |
ΔrH0 = -615.438
Δ rH298 = -613.474 |
2013-10-11 |
N + NH → H + N2
T(K): 10-500 Formula: Modified Arrhenius equation Δ rH0 = -615.438 Δ rH298 = -613.474 2013-10-11 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NH
→
H + N2
|
10-500 | 3.56e-11 | 5.00E-11 | 1.00E-1 | 0.00E+0 | 2 | 6 | lognormal |
N + NH → H + N2
T(K) = 10-500 α = 5.00E-11 β = 1.00E-1 γ = 0.00E+0 F 0 = 2 g = 6 Type uncert: lognormal Method: Reviews and Evaluations |
|
Method: Reviews and Evaluations
|
|||||||||
Other database : OSU T(K) = 10-280
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NH
→
H + N2
|
10-280 | Modified Arrhenius equation |
ΔrH0 = -615.438
Δ rH298 = -613.474 |
2009-03-27 |
N + NH → H + N2
T(K): 10-280 Formula: Modified Arrhenius equation Δ rH0 = -615.438 Δ rH298 = -613.474 2009-03-27 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NH
→
H + N2
|
10-280 | 5.00e-11 | 5.00E-11 | 0.00E+0 | 0.00E+0 | 1.25 | 0 | lognormal |
N + NH → H + N2
T(K) = 10-280 α = 5.00E-11 β = 0.00E+0 γ = 0.00E+0 F 0 = 1.25 g = 0 Type uncert: lognormal Method: Estimation |
|
Method: Estimation
|
|||||||||
| Reference | |||||||
|---|---|---|---|---|---|---|---|
| Other database : OSU | |||||||
|
Origin: Other database : OSU
Authors: Volume,Page: Year: DOI: - Download citation: |
|||||||
Hebrard, E. et al (2009) T(K) = 50-200
Show/Hide those values
| Channels | |||||
|---|---|---|---|---|---|
|
N + NH
→
H + N2
|
50-200 | Modified Arrhenius equation |
ΔrH0 = -615.438
Δ rH298 = -613.474 |
2012-07-27 |
N + NH → H + N2
T(K): 50-200 Formula: Modified Arrhenius equation Δ rH0 = -615.438 Δ rH298 = -613.474 2012-07-27 |
Show details
See/hide publications
|
k(
)
cm3s-1 |
Details | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
N + NH
→
H + N2
|
50-200 | 3.49e-11 | 1.91E-10 | 5.00E-1 | 0.00E+0 | 3 | 100 | lognormal |
N + NH → H + N2
T(K) = 50-200 α = 1.91E-10 β = 5.00E-1 γ = 0.00E+0 F 0 = 3 g = 100 Type uncert: lognormal Method: Reviews and Evaluations Description: Maximum collision rate. Used in Hébrard et al. [2009] |
|
Method: Reviews and Evaluations
Description: Maximum collision rate. Used in Hébrard et al. [2009] |
|||||||||